Frequently Asked Questions
The TM Flow device offers numerous benefits for medical practices, including non-invasive and accurate testing, early detection of autonomic and arterial dysfunctions, and comprehensive diagnostics that reduce the overall cost of patient care. Additionally, the device’s tests are easy to bill with multi-code diagnostics, making it a valuable investment for enhancing patient care and optimizing practice efficiency. It also supports compliance with standards of care recommended by leading health organizations.
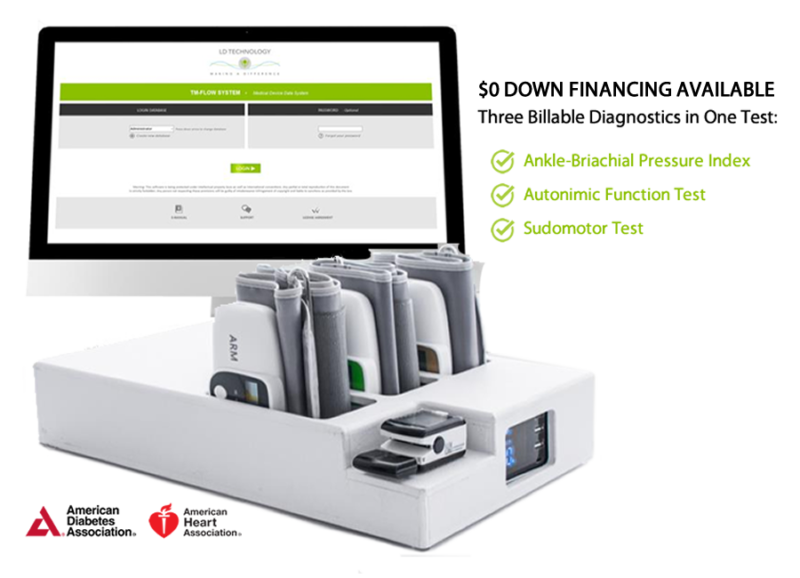
Yes, the TM Flow device is covered by Medicare and most private pay carriers. Specific CPT codes, such as 95921 for cardiovagal innervation and 93923 for ankle-brachial pressure index, ensure that the diagnostics are easy to bill. Coverage and reimbursements may vary depending on the Medicare locality, but the comprehensive diagnostics offered by the TM Flow device are widely recognized and supported by insurance providers.
Autonomic nervous system testing is crucial for diabetes and cardiovascular patients because it helps in the early detection of autonomic neuropathy, which is a common complication in these conditions. Early detection through ANS testing allows for timely intervention and management, reducing the risk of further complications. The American Diabetes Association recommends ANS testing as a standard of care for patients with Type 1 and Type 2 diabetes, highlighting its importance in effective diabetes management.
The TM Flow device is an advanced, non-invasive diagnostic tool designed to perform a series of tests that aid in the identification and early detection of autonomic nervous system (ANS) and arterial dysfunctions. It provides quantitative assessments of the ANS, helping to distinguish between early and late stages of autonomic neuropathy. The TM Flow device is particularly valuable for cardiovascular and diabetic autonomic neuropathy (DAN) testing, making it an essential tool in diabetes management and cardiovascular assessments.
When billing for ANS Testing, here a few key things to keep in mind in terms of which code to use:
Code 95924: should be reported only when both the parasympathetic function and the adrenergic function are tested together with the use of a tilt table. To report autonomic function testing that does not include beat‐to‐beat recording, or for testing
without use of a tilt table, use 95943.
CPT 95921: Testing of autonomic nervous system function; cardiovagal innervation (parasympathetic function), including two or more of the following: heart rate response to deep breathing with recorded R‐R interval, Valsalva ratio, and 30:15 ratio
CPT 95922: Vasomotor adrenergic innervation (sympathetic adrenergic function), including beat‐to‐beat blood pressure and R‐R interval changes during Valsalva maneuver and at least 5 minutes of passive tilt Do not report 95922 in conjunction with 95921
CPT 95924: combined parasympathetic and sympathetic adrenergic function testing with at least 5 minutes of passive tilt Do not report 95924 in conjunction with 95921 or 95922
CPT 95943: Simultaneous, independent, quantitative measures of both parasympathetic function and sympathetic function, based on time‐frequency analysis of heart rate variability concurrent with time‐frequency analysis of continuous respiratory activity, with mean heart rate and blood pressure measures, during rest, paced (deep) breathing, Valsalva maneuvers, and head‐up postural change Do not report 95943 in conjunction with 93040, 95921, 95922, 95924
CPT 95923: sudomotor, including one or more of the following: quantitative sudomotor axon reflex test (QSART), silastic sweat imprint, thermoregulatory sweat test, and changes in sympathetic skin response
RSS FEED
- The TM Flow is a highly reimbursable diagnostic in Portland, OR
- Pain Clinics in Albuquerque, NM Adopt the TM Flow as Gold Standard
- Neuropathy in Philadelphia, PA can be detected with the TM Flow device
- Pain Management Clinics in Dallas, TX are Using the TM Flow to Diagnose Symptoms
- Unlocking the Potential of ANS Testing: Insights for Healthcare Providers
- Neuropathy clinics in Savanah, GA use the TM Flow
- Come to Charleston, WV to Get a TM Flow
- How the TM Flow will maximize your physician practice in Boise Idaho
- Doctors in Pittsburg, PA are using the TM Flow for the treatment of neuropathy
- Which Patients Require ANS Testing?